A simple and sensitive silver-staining method for detecting AFLP markers in
fungi
FGN 47:101-102
Shaobin Zhong and Brian J. Steffenson, Department of Plant Pathology, North Dakota State University
Fargo, ND 58105
A simple and fast silver-staining method is described for detecting amplified fragment length polymorphism (AFLP) markers separated in denaturing polyacrylamide gels. The silver-staining method produced a good degree of resolution and sensitivity and could be widely applicable in AFLP analyses of fungi.
The AFLP technique (Vos et al. 1995. Nucleic Acids Res. 23:4407-4414) has been widely used for genetic studies in many organisms including fungi. However, most of the procedures used to detect AFLP bands involve isotopes such as 32P and 33P that are relatively expensive, inconvenient to handle, and a potential health hazard. Here we describe a simple and fast silver-staining procedure that can be used to detect AFLP markers in fungi.
The fungi used in our experiments included four Cochliobolus species (C. heterostrophus, C. sativus, C. carbonum and C. victoriae) and the barley leaf rust pathogen, Puccinia hordei. DNA extraction from the Cochliobolus species and P. hordei was according to the procedure described by Yoder (Genetics of Plant Pathogenic Fungi. pp.93-112) and Weiland (Fungal Genet. Newsl. 44:60-63), respectively.
AFLP analysis was conducted using a method modified from Vos et al. (Nucleic Acids Res. 23:4407-4414). The AFLP core reagent kit (Life Technologies, Inc., Grand Island, NY) was used to prepare template DNA for PCR. Primers with one selective base were used in pre-selective amplification. Selective amplification was performed using primers containing two selective bases, and a 10-fold dilution of the pre-selective amplification PCR products was used as templates.
Selectively amplified PCR products were separated in a denaturing polyacrylamide gel (7M Urea, 0.5 x TBE, 6% 19 acrylamide:1bis, 40 ul TEMED and 10% ammonium persulfate) and were detected using the DNA silver-staining system (Promega, Madison, WI). Glass plate treatments, gel preparation, and silver-staining were according to the manual provided by the supplier (Promega, Madison, WI).
All images of the silver-stained gels were scanned into a computer with a scanner. To preserve the gel on paper, the glass plate with the gel was dipped into a 2% NaOH solution for 30-50 min. The gel was transferred onto a chromatography paper and air dried overnight.
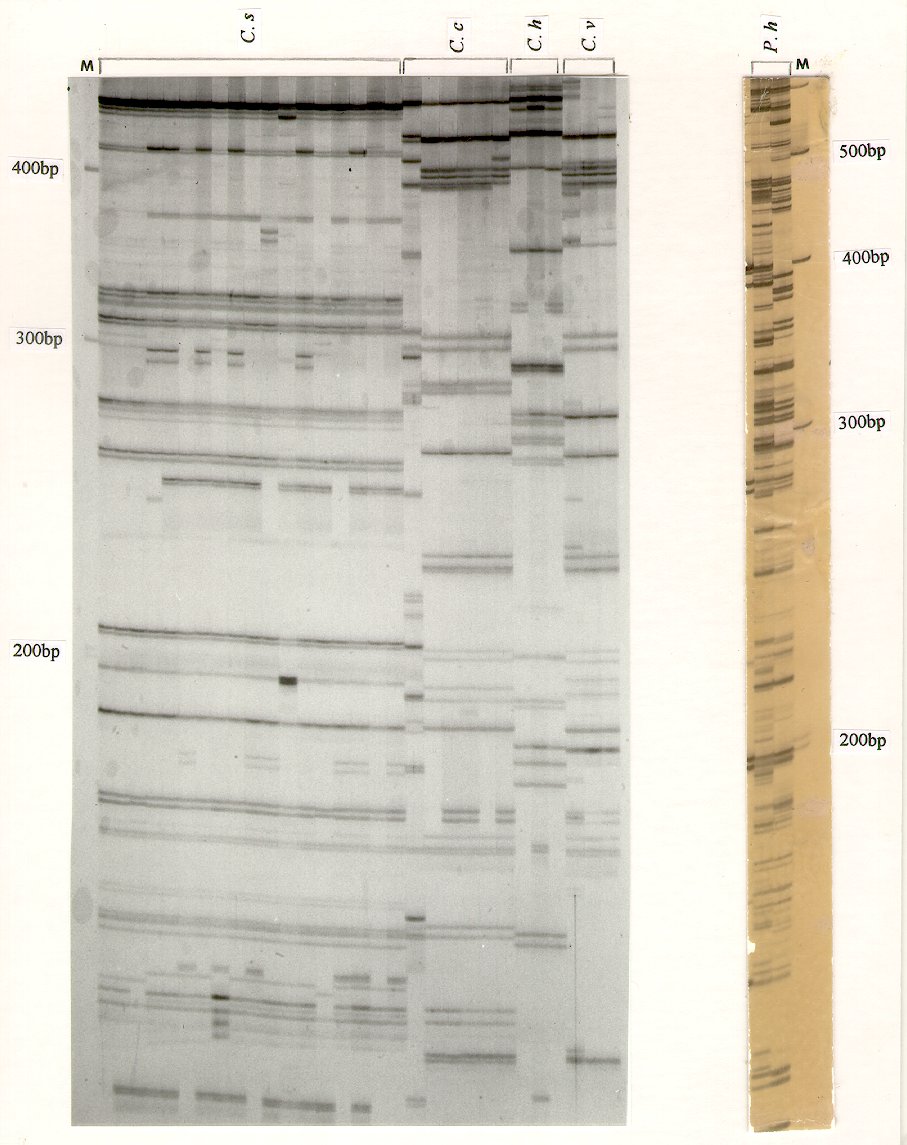
Thirty to sixty-five AFLP markers of size between 80 bp and 800 bp were detected for Cochliobolus species (Fig.1a) and up to eighty bands were detected for P. hordei (Fig.1b). The procedure is simple and fast (only 2 hours for the silver staining steps) and produces bands with good resolution and sensitivity; it compares favorably to isotopic procedures. Additionally, the AFLP bands of interest can be excised directly from the silver-stained gel and cloned into a plasmid. This silver-staining AFLP method should find wide applications for mapping genes of interest, characterizing population structures, and differentiating races and isolates in plant pathogenic fungi.

Figure 1. (a)Detection of AFLP markers from Cochliobolus species using the DNA silver-staining system. The figure shows the DNA fragments from about 110 bp to 420 bp. The primers used were E-AA (5'-GACTGCGTACCAATTCAA-3') and M-CC (5'-GATGAGTCCTGAGTAACC-3'). M, DNA size marker; C. s, C. sativus; C. h, C. heterostrophus; C. c, C. carbonum; C. v, C. victoriae.
(b). Detection of AFLP markers from Puccinia hordei using the DNA silver-staining system. The primers used were E-AG (5'-GACTGCGTACCAATTCAG-3') and M-CA (5'-GATGAGTCCTGAGTAACA-3'). The figure shows the DNA fragments from about 120 bp to 620 bp. M, DNA size marker; P. h, P. hordei. i
Return to the FGN 47 page
Return to the main FGN page
Return to the FGSC main page
Last modified 8/3/00 KMC